Structural steel has been a dominant building material for more than 100 years. Although steel is not considered a particularly remarkable material today, Vaclav Smil’s book, Still the Iron Age, illustrates how important iron and steel have been and continue to be in industrialized societies. For a structural engineer working on historic renovations and adaptive reuse of pre-war buildings, working knowledge of the history, development, and metallurgy of structural metals is necessary for the engineer to be effective and efficient.
The three primary ferrous metals used in building construction from approximately the 1850s to the 1920s were cast iron, wrought iron, and structural steel. All three materials are man-made metals (alloys) whose primary ingredient is iron. The industrial revolution of the 18th and 19th centuries brought iron making technology to an advanced state where cast iron and then wrought iron could be mass-produced and used, first in transportation and then building projects.
Iron technology was used and developed predominantly in Europe, China, the Middle East, and India. The basic process consisted of smelting mined iron ore (naturally occurring deposits of iron-rich minerals) and forging iron bars from the resulting “bloom” or sponge-like mass of iron, separating the resulting slag and impurities. Smelting was done in a furnace known as a bloomery, consisting of a chamber or pit with masonry walls and an exhaust stack. The fuel typically consisted of charcoaled wood or coal, which had a dual purpose of creating heat and providing carbon. When the carbon combined with pure iron, the process created the first ferrous metals like wrought iron.
The introduction of air or oxygen via bellows and pipes, called tuyeres, was added to increase the heat within the furnace. Early development was limited by the availability of fuel and the ability to achieve high temperatures. However, the technology slowly advanced and came to fruition with the industrial revolution, first in England and then in other parts of Europe and the United States. The bloomery was eventually replaced by a blast furnace – blast referring to a blast of air (oxygen) and coke as a fuel instead of charcoal. Coke was a fuel made from bituminous coal that was first heated to reduce water content and impurities – a process called “coking.” The resulting coke burned better and had a higher carbon content. This advanced technology produced metal in liquid form at the bottom of the furnace, known as “pig iron.” The term came from the collected metal shapes of molds and bars that resembled a litter of pigs. Pig iron is basically a cast iron or alloy of iron with high carbon content. Wrought iron, cast iron, and, eventually, steel were made by further refining pig iron.
Cast iron is an alloy of iron, with a high carbon content, cast into molds to create various shapes; it has good compressive strength and low tensile strength. Wrought iron is a more malleable or workable (hence the name “wrought”) alloy of iron with low carbon content and good tensile and compressive strengths. Both metals were used in early building structures, particularly industrial buildings in England, to replace and span farther than the heaviest timbers available. Steel, which is also an alloy of iron with low carbon content and other elements such as manganese, silicon, sulfur, and phosphorus, eventually replaced cast iron and wrought iron. Steel was known and available in limited quantities in the early 1800s and became commercially available after Henry Bessemer developed an efficient process for producing steel, which he patented in 1856. This process, called the Bessemer process, was improved by others and eventually replaced by the open-hearth process. Cast iron was used predominately in the early to mid-1800s for structural members and eventually relegated to columns and decorative uses due to its high compressive strength but brittle nature. Wrought iron was used in the latter half of the 1800s and replaced cast iron for flexural members due to its superior tensile properties.
By the late 1800s, structural steel began to take over the market for structural metals and was stronger than wrought iron, both in tensile and compressive properties. In the building industry, particularly from the Chicago School of Architecture, traditional heavy masonry buildings began to grow taller and introduce interior skeletal framing, first with cast-iron columns and wrought-iron beams and eventually with full skeletal steel framing. The “Chicago School of Architecture” was a style of architecture that came out of Chicago in the late 1800s and early 1900s. The architects, engineers, and builders, experiencing a building boom due to market forces and the rebuilding from the aftermath of the 1871 Great Chicago Fire, began to experiment with new materials and techniques. This was the beginning of the common skeletal frame type of construction. The steel manufacturing industry grew, and each steel producer used their own formulations, resulting in the finished product’s variability.
Individual companies developed allowable load tables for products made in the late 1800s and early 1900s. In 1900, the American Society of Testing Materials (ASTM) developed standards for structural steel such as ASTM A9, Specification for Steel for Buildings. These standards defined minimum requirements for the steel materials used in these applications, bringing uniformity to the varying standards published by the individual producers of the time. In 1939, the A9 standard for buildings was consolidated into ASTM A7 (the bridge standard up to that point), and the two standards remained combined until 1960, when the ASTM A36 standard was issued. This standard became the dominant standard for steel buildings until the 1990s. In 1921, the American Institute of Steel Construction (AISC) was founded to bring consistency to the design and construction standards for structural steel used in building construction, and the first AISC Standard Specification for Structural Steel for Buildings followed in 1923.
Metallurgical Differences
Cast iron contained a larger percentage of carbon (2% to 4%) and was produced by melting pig iron mixed with scrap metal in a “cupola” furnace. Though easily cast, it was brittle and not malleable. On the other hand, wrought iron is almost pure iron with a very slight amount of carbon (0.2% to 0.35%). It was manufactured by melting pig iron in a puddling furnace where many impurities from the pig iron were removed. The resulting product was an iron alloy with a glass-like fibrous slag that is malleable and has good tensile properties and good corrosion resistance. The distribution of the slag fibers resulted in tensile strength and ductility higher in the longitudinal or rolling direction (Figure 1). By the early 1900s, steel completely replaced cast iron and wrought iron for structural framing. It is an alloy of iron with a carbon content of less than 2% (mild structural steel has a carbon content more in the magnitude of 0.3% to 0.6%). It has a crystalline grain structure on a microscopic level, is ductile, and, unlike wrought iron, is more susceptible to corrosion. However, the relatively low carbon content and small amounts of other elements give it great strength and ductility.
Field and Laboratory Investigations
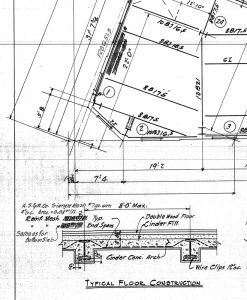
Investigating old buildings is like reading a detective novel. Much the way a detective first visits a crime scene, makes field observations and notes, collects forensic evidence, and conducts laboratory testing, an engineer performs the same tasks on existing framing conditions. The first step is an initial walkthrough to determine the existing structure’s general layout and get the lay of the land. Interviews with building superintendents can yield valuable information on previous renovations or descriptions of hidden conditions, or, in some lucky instances, rolls of original drawings tucked away in a corner (Figure 2). A detailed survey of the framing is then conducted. A keen eye can pick up clues like a “Pencoyd” or “Carnegie” stamp on the side of an old beam (Figure 3).
Back in the office, the wealth of information in engineering literature can be used to determine allowable stresses, locate old load tables, and determine the limits and design methodologies at the time of construction. Sources like the American Institute of Steel Construction (AISC) Design Guide 15, Rehabilitation and Retrofit, and the AISC Engineering Journal Field Welding to Existing Steel Structures by David Ricker, First Quarter/1988, are invaluable resources for determining the properties and weldability of structural steel. Other sources like Kidder Parker’s, Architects’ and Builder’s Handbook, now in the public domain, provide original load tables, historical details, and guidance on older systems’ methodologies. Knowledge of typical historical details can help in a field survey as well. For example, cast-iron columns often had integral cast seats or lugs for supporting timber, wrought iron, or steel beams (Figure 4). Knowledge of this detail makes it easy to spot cast-iron columns. Drilling holes in the cast iron to ascertain its thickness can allow for a capacity calculation from original load tables and formulas based on the column’s height.
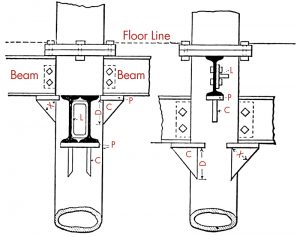
Figure 4. A typical beam to cast iron column detail. Note beams bear on cast lugs. From Kidder/Parker Architects’ and Builders’ Handbook.
Ultimately, laboratory testing of samples from the metal will provide the most definitive proof of older metals’ metallurgy and weldability. Some quick “sanity” checks are also useful for comparative and validation purposes. For example, if one knows the initial loading and size of a beam and then back calculates the actual stresses, a low level of stress of say 8 to 10 kips per square inch (ksi) would indicate the metal is most likely wrought iron, rather than structural steel which would most likely be 16 to 18 ksi. Additionally, if one found an I-beam of unknown material but had, say, a 1-inch-thick flange for a 10-inch-deep beam, one could deduce that the beam is likely wrought iron since no 10-inch-deep steel beams had flanges that thick.
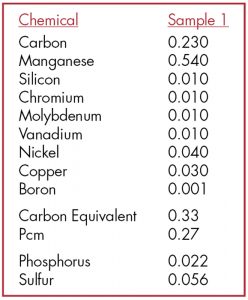
Figure 5. Sample from a laboratory analysis showing the results of a chemical analysis of a steel beam from ca. 1910.
Laboratory testing is the best method for definitively determining the material, its weldability, and its yield strength. Specimens removed from the field at innocuous locations, such as the flange at the end of a simply supported beam, can be sent to a metallurgist for laboratory testing. Ricker provides guidance on this and determines the equivalent carbon content for steel weldability (Figure 5). Steel, with lower carbon, particularly after the 1920s, is generally very weldable. Cast iron and wrought iron (though weldable) are not reliable for welding purposes. It is generally recommended to strengthen and connect to those members by mechanical means. Another test, inherently qualitative, is the inexpensive spark test. A specimen is brought into contact with a grinding wheel, and the resulting stream of sparks observed can confirm the presence of carbon (Figure 6). Pure iron (e.g., wrought iron) results in smooth spurts of sparks, whereas the carbon in steel produces forked sparks; the more significant the carbon content, the more numerous the forks.
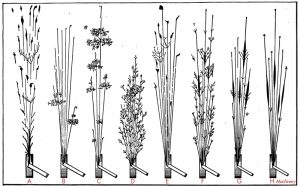
Figure 6. Diagram of sparks showing branching and forking. Part A shows wrought iron, and Parts B through E shows various irons or steels. The presence of carbon is relative to the branching or forking of the sparks. From Iron and Steel by Erik Oberg and Franklin Day Jones, 1918.
Repairs, Alterations, and Renovations
Historic metals are typically encountered when repairing, altering, and renovating historic buildings. A common repair scenario involves strengthening due to rusting or corrosion. Corrosion, an electrochemical reaction (oxidation and reduction) that results in the expansion of iron alloys, particularly steel, causes sectional loss of members. The reaction requires water, and thus, unprotected or inadequately protected metal is highly susceptible to corrosion.
Strengthening techniques of existing metal structures can be accomplished by shortening the span of the existing members, adding new structural members, replacing the existing structural members, post-tensioning (external prestressing) the existing members, and/or enlarging the structural member’s section by welding new reinforcing steel or by introducing composite action.
In adaptive reuse of older structures, new performance requirements often compel the addition of reinforcing material to increase load-carrying capacity, to restore areas eroded by corrosion, to strengthen fire-weakened members, or perhaps alter the appearance of a member by changing its shape for aesthetic reasons.
In Conclusion
Identifying the material through visual inspections, confirming details and original design methodology via consulting original texts and older codes, and confirming yield strengths and weldability via laboratory testing are the tools needed for successfully dealing with historic metals.■