Improving Performance of Concrete Structures
Corrosion of steel reinforced concrete affects everyone, leading to expensive repairs and intensive maintenance programs. Budgeting of repairs and maintenance programs becomes difficult when structures do not meet their intended life cycle requirements. Corrosion of reinforcing steel in concrete structures leads to concrete failure, impacting the public with delays and detours. The Federal Highway Administration’s National Bridge Inventory records reflect that less than 16% of existing U.S. bridge decks utilize corrosion resistant reinforcing steel. Beyond bridge decks and highway infrastructure, corrosion protection of concrete reinforcement is an even smaller percentage. Preservation of assets by using zinc-coated reinforcing steel can be a cost-effective solution to improving the life cycle and durability of concrete structures.
Maintenance programs often include Cathodic Protection by attaching zinc anodes to steel reinforcement to delay further damage of existing corrosion. This has proven to be an effective means of preventative maintenance in different types of structures. Results can be achieved over the lifetime of the structure without the cost of expensive rehabilitation by using zinc-coated reinforcement from the beginning. With recorded installations prior to the 1960s, hot-dip galvanized reinforcement in concrete is a proven performer. Multiple case studies indicate that these mitigation techniques result in structures lasting decades.
The galvanizing industry continues to innovate and expand as process technologies and market conditions improve. Hot-dip galvanized steel is a widely used coating in atmospheric conditions for automotive, transportation, construction, energy, and power markets. The use of hot-dip galvanizing in concrete can be traced back decades in different applications. Bridge decks, installed as far back as the 1970s, that are exposed to extreme conditions can be found performing well. Structures from around world, such as the Sydney Opera House, Lotus Temple in India, New York’s new NY Bridge, and Canada’s AutoRoute 40 in Montreal, have successfully used hot-dip galvanized reinforcement. In addition to traditional hot-dipped (ASTM A767) galvanizing, reinforcing bar can now also be coated in a continuous process (ASTM A1094). This innovative process takes the advanced technology used in the sheet steel industry and applies it to reinforcing steel. Galvanized coatings have always provided both barrier and sacrificial protection for steel. With this technology, the bars can also be formed after coating without cracking or peeling of the zinc layer. The automated process allows the development of a pure zinc alloy rather than a zinc-iron alloy that, in heavier thicknesses, can become brittle. The continuous galvanized rebar process also provides a paintable substrate for dual coated reinforcement (ASTM A1055). The ASTM A1055 specification has been modified to allow two types of zinc alloy coatings, thermal spray (type 1) or as applied by ASTM A1094 (type 2).
Zinc coatings passivate very quickly when exposed to fresh concrete, which enhances the long-term corrosion protection of the galvanized reinforcements during years of service. The initial passivation of a zinc coating, when embedded in concrete, occurs within hours and is affected by the chemistry of the surface layer.
The relationship between cement alkali content and zinc corrosion rate is important to understanding the initial passivation of galvanized coatings in concrete. The pH of cement in contact with the galvanized coating controls the formation of a compact and adherent layer of calcium hydroxyzincate (CHZ), a compound that passivates the surface of the zinc coating from further reaction with the concrete. The threshold for passivation of zinc in concrete pore solutions is at a pH of between 12.8 and 13.2 +/- 0.1. PH levels greater than 13.2 do not develop in concrete pore solutions during the first few hours if sulfate is used as a settling regulator, or enough alkaline sulfates are present. The passivation layer develops during the first few hours after mixing when the pH of the concrete solution is lower than 12.8 +/- 0.1. If pH is between 12.8 and 13.2, the layer develops slowly and the galvanized coating may continue to react until the passivating layer is formed. In any case, regardless of the pH level of the concrete, the presence of a pure zinc layer is key to the rapid formation of a compact passivating film of CHZ on the galvanized reinforcement.
The corrosion of reinforcement occurs while in service when aggressive species (carbonation front or chloride ions) reach the reinforcement. These aggressive species have to disrupt the physical barrier of the CHZ film to initiate corrosion of galvanized reinforcement. Carbonation lowers pH from highly alkaline to around neutrality (pH 7), where the rate of Zn corrosion is very low. As a result, galvanized reinforcement does not generally corrode in carbonated concrete. Chlorides are the more aggressive ions for reinforced concrete and are the most frequent cause of reinforcement distress. Chloride ions come from the raw construction materials, marine environments, or deicing salts. Zinc is attacked by chloride ions but has a higher threshold value for corrosion to initiate than bare steel. That is, the concentration of chloride ions needed to start corrosion of zinc is up to four times higher than the concentration required to start corrosion of black steel. The overall behavior depends on the source of the chloride ions, the state of the galvanized surface (including protection afforded by Zn corrosion products), and the degree of protection provided by the concrete cover.
The corrosion of steel in concrete can be viewed as developing through a two-stage mechanism: initiation and propagation. Efforts to achieve long-term durability of reinforced concrete have been mostly directed at delaying initiation of corrosion of the reinforcement, i.e. postponing as long as possible the start of the propagation stage. The presence of a pure zinc layer on the surface of the steel reinforcement is the best way to delay the onset of corrosion of the reinforcement. The tenacious passivating film of CHZ is the first line of defense. The pure zinc layer will then corrode uniformly at less than one-tenth the corrosion rate of the base steel, thereby extending the onset of corrosion of the steel reinforcement. It should also be noted that the zinc corrosion products migrate away from the corrosion site and help densify the concrete surrounding the reinforcement, further delaying the onset of corrosion and also increasing bond strength.
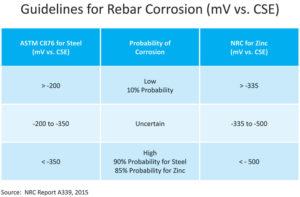
Recent research from the National Research Council of Canada reviewed how zinc-coated reinforcing steels perform favorably compared to uncoated reinforcing steels. In heavily chloride-contaminated concrete, galvanized steel was found to have 5-10 times lower corrosion rates than carbon steel, depending on environmental exposures. The NRC report, Condition Assessment and Corrosion Mitigation of Galvanized Steel Reinforcement in Concrete Structures, presents the corrosion performance of galvanized steel as compared to carbon steel. It also explores the corrosion mitigation strategies for further improving its corrosion resistance to extend the service life of concrete structures. The three-year study included a comprehensive program to obtain corrosion data that represent a broad spectrum of corrosion states of galvanized steel in concrete structures. The corrosion rates spanned over five orders of magnitude, from a passive state to low, moderate, and high corrosion rates, by combining the key corrosion parameters including environmental exposure and chloride content as well as concrete type. These data were used for both corrosion performance analysis and statistical analysis for the development of model/guidelines for corrosion condition assessment. It also included a field survey that was conducted on two highway bridges, and the collected half-cell potential data were used to validate the developed condition assessment model/guidelines.
Another recent University of Waterloo thesis, Evaluation of the Corrosion Behaviour of Continuously Galvanized Rebar, reviews autopsied concrete slabs that contained several varieties of black and galvanized reinforcement after 450 days of exposure. Electrochemical test measurements were made throughout the life of the project. For the corrosion potentials, ASTM C876 which characterizes black steel corrosion in concrete was referenced. These were taken bi-weekly over the 450-day period for three types of concrete samples: sound (non-cracked), transverse cracked, and longitudinally cracked samples. The electrochemical readings were interpreted using the NRC report which gave the threshold values for corrosion of zinc in concrete. Results demonstrate that zinc coated reinforcement performs better than conventional uncoated reinforcement in all cases.
Zinc-coated reinforcing steels are an optimal way of preserving concrete assets with their proven performance, durability, and cost effectiveness. Hot-dip galvanized reinforcing steel is widely available throughout North America. Continuous Galvanized Reinforcement (CGR) will be available in North America in early 2017 and is currently available in Xiamen, China, and in Dubai, UAE.▪
References
Yeomans, S. R. (2004). Galvanized Steel Reinforcement in Concrete, Elsevier Ltd, Oxford.
Tan, Z.Q., Hansson, C.M. (2008). Effect of surface condition on the initial corrosion of galvanized reinforcing steel embedded in concrete, Corrosion Science 50, 2512-2552.
Kinstler, T.J. (2002). Research and Update on Galvanized Reinforcing Steel.
Ogunsanya, I. (2016). Evaluation of the Corrosion Behaviour of Continuously Galvanized Rebar. Retrieved from Waterloo’s Institutional Repository, https://uwspace.uwaterloo.ca/handle/10012/10943
Zhang, J. Baldock, B. (2015). Condition Assessment and Corrosion Mitigation of Galvanized Steel Reinforcement in Concrete Structures, NRC-CNRC, Ottawa.
ASTM A767 / A767M-16, Standard Specification for Zinc-Coated (Galvanized) Steel Bars for Concrete Reinforcement, ASTM International, West Conshohocken, PA, 2016, www.astm.org
ASTM A1094 / A1094M-16, Standard Specification for Continuous Hot-Dip Galvanized Steel Bars for Concrete Reinforcement, ASTM International, West Conshohocken, PA, 2016, www.astm.org
ASTM A1055 / A1055M-10e1, Standard Specification for Zinc and Epoxy Dual-Coated Steel Reinforcing Bars, ASTM International, West Conshohocken, PA, 2010, www.astm.org
Federal Highway Administration: Home, www.fhwa.dot.gov/bridge/nbi.cfm